Abstract-- Calcium hydroxyapatite was produced from pork
bones through a three step procedure including pre-pyrolysis, chemical
impregnation with K2CO3 and pyrolysis. The effect of
pyrolysis temperature on the porosity development of the material was
investigated. The specific surface area (SSA) decreases as charring temperature increases. It is observed that the chemical treatment with K2CO3
is
inefficient at mild pyrolysis conditions, in the 550-
Keywords-- Hydroxyapatite; Bone char; Chemical activation; Physicochemical characterization;
TG-MS.
I. INTRODUCTION
Owing to its desirable structural stability, ionic substitution ability
and acid–base properties, the use of hydroxyapatite (Ca10(PO4)6(OH)2)
(HAp) has recently increased. HAp is a versatile material and recently it
has been successfully used for the adsorption of organic pollutants (Ciobanu et al., 2014) and metals (Kim and Lee, 2014; Sicupira et
al., 2014) from liquid effluents, as a support
in photocatalytic systems (Chun et al., 2014) and also in electrochemical (Goodman et al., 2013) and biomedical applications (Sobczak-kupiec et al., 2013).
Many methods of synthesizing
HAp have been reported, such as solid-state reaction, sol–gel, wet synthesis
and hydrothermal methods (Guo et al., 2013; Salviulo et al., 2011). However, the procedures employed
were relatively complicated and it has been proven difficult to obtain
controlled morphology with these methods. Hard tissues in animals represent an
abundant source of natural HAp (Larsen et al., 1993; Singh,
2012). If adequate methods are developed,
the use of waste animal bones is a promising alternative choice for the
production of HAp-based porous materials.
Pyrolysis constitutes one of
the most reliable treatment methods for the animal bones left over. The solid
fraction obtained (char) contains about 70–76 wt% calcium hydroxyapatite Ca10(PO4)6(OH)2,
9–11 wt% carbon and 7–9 wt% CaCO3. The apatite crystals in bones can
act as natural template for the formation of abundant pores. Furthermore, this
process could be economically feasible as well as environmentally friendly.
Difficulties have been
reported in the production of materials based on natural HAp with large
specific surface area (SSA). Doostmohammadi et
al. (2012) reported a surface area value of
2.2 m2/g, obtained by the charring of bovine bones in air atmosphere
at
Chemical activation
represents an alternative for improving the textural and surface properties of
bone char. It requires lower temperature and results in higher yield than
physical activation only (Lillo-Ródenas et al., 2003; Phan et al.,
2006). In a
previous work our research group (Iriarte-Velasco et al., 2015a; 2015b) concluded that the chemical activation with K2CO3
significantly enhances the textural properties of bone char. However, there
are still important gaps in the fundamentals of the process, regarding the
optimization of preparation conditions and the reaction mechanisms that take
place. The activation
temperature is one of the most important factors. Sobzak et
al. (2009) investigated the textural
properties of pig bone calcined in air atmosphere. Calcinations in the 650 –
900 ºC range caused variations in SBET values in the 44 – 3 m2/g
range.
In this study a porous
material, mainly composed of natural HAp, has been produced by using pork bones
as raw material. To the best of the authors’ knowledge, there is no study in
the literature regarding the effect of the pyrolysis temperature on
the production of natural HAp through the activation of pork bones with K2CO3. The specific objectives of the
present work can be described as follows: i) investigate the effect of
pyrolysis temperature on porosity development within the bone char; ii)
identify the reactions that occur during the chemical activation process; and
iii) determine how such reactions contribute to the evolution of porosity and
specific surface area.
II. METHODS
A. Preparation of bone char
Pork chop bones were pre-pyrolysed at chemical
treatment (BCO). K2CO3-treated samples were coded as BCK.
B. Characterization of bone char
A porosimeter (ASAP 2010, Micromeritics) was used to determine the
textural properties, by means of nitrogen adsorption/desorption at 77 K. BET
surface area, and pore area and volume were determined. Micropore surface and
volume were measured from density functional theory (DFT), while values in the
mesopore and macropore ranges were measured based on the Barrett, Joyner &
Halenda method (BJH).
In order to investigate the
reactions occurring during the activation process, thermogravimetric analysis
(TG) coupled to mass spectrometry (MS) was conducted. TG analyses were
performed with a Setsys Evolution (Setaram) thermal analyser. About 70 mg of
impregnated samples were put into the alumina crucible and heated under helium atmosphere from room temperature up to
III. RESULTS AND DISCUSSION
A. Specific surface area and volume
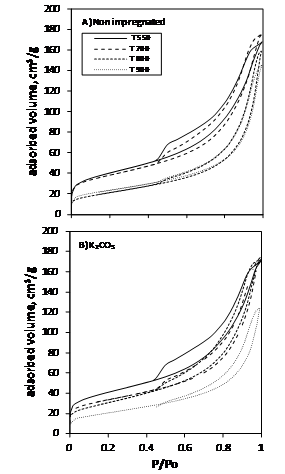
Figure 1 shows the nitrogen adsorption desorption curves. All isotherms exhibited the presence of a hysteresis loop, which is a characteristic feature of the Type IV isotherm. The downward displacement of the curve with the increased calcinations temperature was indicative of the collapse of porous structure. For chemically activated samples, this effect was intensified at the highest temperatures investigated.
Figure 2
shows the total specific surface area (SSA) and pore volume (Vp) of
biochars. Figure 3 exhibits the fractional surface areas. Complementary
textural data are presented in Table 1. The following regions have been
considered, based on the location of valleys in the pore size distribution (not
shown): micropores, SSAI (Dp<1.7 nm); small mesopores,
SSAII (1.7<Dp<5.0 nm) and the sum of large
mesopores and macropores SSAIII (Dp>5.0 nm).
Figure
1. Nitrogen adsorption-desorption isotherms.
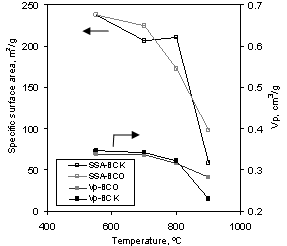
Figure 2. Influence of
pyrolysis temperature on the specific surface area (SSA) and pore volume (Vp)
of K2CO3 treated (BCK) and non-treated (BCO) bone char.
The maximum SSA (237 m2/g)
was obtained for non-treated sample pyrolysed at mild temperature, 550ºC. At
that temperature, there are no significant differences between treated and
non-treated (BCO) samples. A significant variation in SSA is observed as temperature
varies. For BCO, specific surface area continu-
Table 1. Textural properties of the
prepared biochars. T, ºC; SBET, m2/g; dp, Å; V, cm3/g.
|
|
T |
SBET |
dp |
VI |
VII |
VIII |
|
BCO |
550 |
142 |
70 |
0.021 |
0.07 |
0.19 |
|
|
700 |
134 |
80 |
0.021 |
0.05 |
0.21 |
|
|
800 |
76 |
107 |
0.017 |
0.03 |
0.22 |
|
|
900 |
83 |
102 |
0.003 |
0.03 |
0.20 |
|
BCK |
550 |
145 |
74 |
0.022 |
0.07 |
0.20 |
|
|
700 |
117 |
89 |
0.017 |
0.04 |
0.23 |
|
|
800 |
115 |
109 |
0.023 |
0.05 |
0.20 |
|
|
900 |
75 |
125 |
n/d |
0.01 |
0.17 |
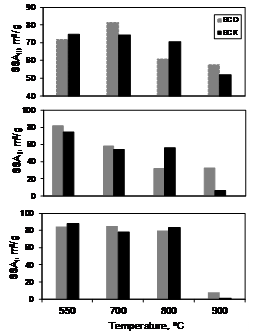
Figure 3. Influence of pyrolysis temperature on the fractional
surface area of non-treated (BCO) and K2CO3 (BCK) treated bone char. r=1
mmol/g.
ously decreases with charring temperature within all the
temperature range investigated (550 to
Several
authors (Deydier et al., 2005) have also reported a dramatic decrease in surface
area of calcined meat and bone meal, from 18 to 2 m2/g, as
temperature was increased from 550 to
For K2CO3-treated
sample, as activation temperature increases (in the 500-
B. Thermogravimetric analysis
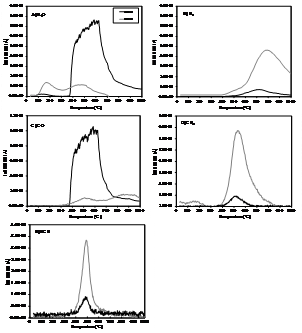
The results of the thermogravimetric
(TG) and differential TG (dTG) analysis of non-treated and K2CO3-impregnated
samples are shown in Figure 4. In the dTG
curves one main peak (with its maximum near
This decomposition process is
gradual and takes place over a range of temperature (Tanaka et al., 1997). The
intense mass loss from around 275 °C to 600 °C is attributed to that process.
Furthermore, the decomposition of collagen has also been associated to this
mass loss (Etok et al., 2007). Figure 4
reveals that mass loss within this range is significantly intensified by K2CO3
treatment.
At above
weight loss was low, about 3 %, which is very similar to the present work.
C. TG-MS analyses
In order to better understand the activation mechanism the following
species were monitored in pyrolysis gases: H2O, H2, CO,
CH4 and HCN. The results are shown in Figure 5. Chemical treatment increases
dramatically the release of H2O and CO whereas that of H2,
CH4 and HCN is diminished. The main release of water starts at
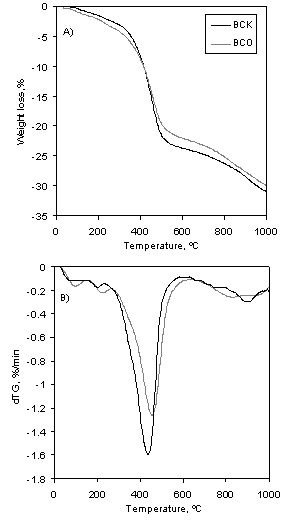
Figure 4. Thermogravimetric analysis of non-impregnated and
chemically impregnated samples of bone char. A) TG curves; B ) dTG curves.
about
![]() . (1) (1)
. (1) (1)
The release
of water observed below
Regarding the
release of CO, McKee (1983) and Lillo-Ródenas et al. (2004) studied the gasification of graphite by alkali metal and found that K2CO3
was reduced in inert atmosphere by carbon as follows:
![]() . (2)
. (2)
Another source of CO is the RWGS reaction (Eq. 3). This reaction has
been reported during the pyrolysis of carbonaceous precursors, and would take
place mainly at temperatures higher than
![]() (3)
(3)
As shown in Figure 5, the release of CO during the activation of
non-impregnated sample occurs in two stages, in the 400-
The release of CO2
is well documented. Both the CaCO3 constituent of bone char and the
incorporated K2CO3 can undergo thermal decomposition (Eqs.
4-5) in the 600-
![]() (4)
(4)
![]() (5)
(5)
The increased release of H2O and CO suggests that K2CO3
can act through different mechanism; i) as dehydration agent; or ii) being
reduced by carbon and releasing CO and thereby increasing the specific surface area
and specific pore volume. Both processes involve gasification reactions and
thus, are expected to have an impact on the textural properties of the prepared
material. Also, when activation temperature reaches the boiling
point of potassium
CH4
and HCN could be formed following a reaction pathway similar to that described
by Robau-Sánchez et al. (2005):
![]() (6)
(6)
The above mechanism implies the
reaction of structural carbon and nitrogen with a source of
The release of water observed belowThe reduced release of these compounds during the charring of K2CO3 treated samples could be attributed to the preferential oxidation of carbon by the activating agent through Eq. (2). In this way, the consumption of much part of the carbon content in bone char would limit the occurrence of Eq. (2) and therefore would explain the limited release of cyanide and CH4 as observed for the sample impregnated with K2CO3.
Figure 5. Temperature dependence of pyrolysis products
for K2CO3 impregnated and non-impregnated bone char.
III. CONCLUSIONS
The results presented demonstrate that chemical treatment with K2CO3
enhances pore development more than physical activation when pyrolysis is
carried out at 800 ºC, mainly in the mesopore and macropore range. At lower
temperatures, in the 550-700 ºC range, the chemical treatment was
inefficient. At higher temperatures, of about pore volume.
The investigation of
pyrolysis gases by TG-MS reveals an increased release of H2O and CO
during the charring of K2CO3-treated bone char. The release
of H2O is related to the decomposition of HAp structure. The release
of CO is partly attributed to the enhanced gasification of carbon in bone char
through its reaction with K2CO3. The obtained results
encourage further investigation into the application of TG-MS as a technique to
control the textural properties of biochars produced by the pyrolysis of waste
material.
REFERENCES
Ayllon, M., M. Aznar,
J.L. Sanchez, G. Gea and J. Arauzo, “Influence of temperature and heating rate
on the fixed bed pyrolysis of meat and bone meal,” Chem. Eng. J., 121, 85-96 (2006).
Chen, W. and B. Lin,
“Hydrogen and synthesis gas production from activated carbon and steam via reusing
carbon dioxide,” Appl. Energy,
101, 551-559 (2013).
Chun, S., S. An, S.
Lee, J. Kim and S. Chang, “Optimization of sulfamethoxazole degradation by
TiO2/hydroxyapatite composite under ultraviolet irradiation using response
surface methodology,” Korean Journal of Chemical Engineering, 31, 994-1001
(2014).
Ciobanu, G., M. Harja,
L. Rusu, A. Mocanu and C. Luca, “Acid black 172 dye adsorption from aqueous
solution by hydroxyapatite as low-cost adsorbent,” Korean Journal of
Chemical Engineering, 31,
1021-1027 (2014).
Deydier, E., R. Guilet,
Dimovic, S., I. Smiciklas,
I. Plecas, D. Antonovic and M. Mitric, “Comparative study of differently
treated animal bones for Co2+ removal,” J. Hazard Mater., 164, 279-287
(2009).
Doostmohammadi, A.,
A. Monshi, R. Salehi, M.H. Fathi, S. Karbasi, U. Pieles and A.U. Daniels, “Preparation, chemistry and physical properties
of bone-derived hydroxyapatite particles having a negative zeta potential,” Mater.
Chem. Phys., 132, 446-452 (2012).
Etok, S., E. Valsami-Jones,
T. Wess, J. Hiller, C. Maxwell, K. Rogers, D.C. Manning, M. White, E. Lopez-Capel,
M. Collins, M. Buckley, K.H. Penkman and S. Woodgate, “Structural and chemical
changes of thermally treated bone apatite,” J. Mater. Sci., 42, 9807-9816
(2007).
Goodman, P.A., H. Li,
Y. Gao, Y.F. Lu, J.D. Stenger-Smith and J. Redepenning, “Preparation and characterization
of high surface area, high porosity carbon monoliths from pyrolyzed bovine bone
and their performance as supercapacitor electrodes,” Carbon, 55, 291-298
(2013).
Guo, X., H. Yan, S.
Zhao, Z. Li, Y. Li and X. Liang, “Effect of calcining temperature on particle
size of hydroxyapatite synthesized by solid-state reaction at room temperature,”
Advanced Powder Technology, 24, 1034-1038 (2013).
Hassan, S.S.M., N.S.
Awwad and A.H.A. Aboterika, “Removal of mercury(II) from wastewater using camel
bone charcoal,” J. Hazard Mater., 154, 992-997 (2008).
Iriarte-Velasco, U.,
J.L. Ayastuy, L. Zudaire amd I. Sierra, “An insight into the reactions
occurring during the chemical activation of bone char,” Chem. Eng. J., 251,
217-227 (2014).
Iriarte-Velasco, U.,
I. Sierra, E.A. Cepeda, R. Bravo and J.L. Ayastuy, “Methylene blue adsorption by
chemically activated waste pork bones,” Coloration Technology, 131, 322-332
(2015a).
Iriarte-Velasco, U.,
I. Sierra, L. Zudaire and J.L. Ayastuy, “Conversion of waste animal
bones into porous hydroxyapatite by alkaline treatment: Effect of the
impregnation ratio and investigation of the activation mechanism,” J. Mater.
Sci., 50, 7568-7582 (2015b).
Jacobs, G. and B.H.
Davis, “Surface interfaces in low temperature water-gas shift: The metal oxide
synergy, the assistance of co-adsorbed water, and alkali doping,” Int. J.
Hydrogen Energy, 35, 3522-3536 (2010).
Kim, Y. and Y.J. Lee,
“Characterization of mercury sorption on hydroxylapatite: Batch studies and microscopic
evidence for adsorption,” J. Colloid Interface Sci., 430, 193-199
(2014).
Larsen, M.J.,
E.I.F. Pearce and J.S. Jensen, “Defluoridation of water at high pH with use of
brushite, calcium hydroxide, and bone char,” J. Dent. Res., 72, 1519-1525
(1993).
Lehman, R.L., J.S. Gentry
and N.G. Glumac, “Thermal stability of potassium carbonate near its melting
point,” Thermochimica Acta, 316, 1-9 (1998).
Lillo-Ródenas, M.A.,
D. Cazorla-Amorós and A. Linares-Solano, “Understanding chemical reactions
between carbons and NaOH and KOH: An insight into the chemical activation
mechanism,” Carbon, 41, 267-275 (2003).
Lillo-Rodenas, M.A.,
J. Juan-Juan, D. Cazorla-Amoros and A. Linares-Solano, “About reactions occurring
during chemical activation with hydroxides,” Carbon, 42, 1371-1375
(2004).
Mckee, D.W., “Mechanisms
of the alkali-metal catalyzed gasification of carbon,” Fuel, 62, 170-175
(1983).
Murillo, Y.S., L. Giraldo
and J.C. Moreno, “Porous materials obtained from chicken and pork bones for the adsorption
of 2,4-dinitrophenol,” Afinidad, 68, 447-452 (2011).
Phan, N.H., S. Rio,
C. Faur, L. Le Coq, P. Le Cloirec T.H. Nguyen, “Production of fibrous
activated carbons from natural cellulose (jute, coconut) fibers for water
treatment applications,” Carbon.,
44, 2569-2577 (2006).
Prabowo, B., K. Umeki,
M. Yan, M.R. Nakamura, M.J. Castaldi and K. Yoshikawa, “CO2–steam mixture for
direct and indirect gasification of rice straw in a downdraft gasifier:
Laboratory-scale experiments and performance prediction,” Appl. Energy, 113,
670-679 (2014).
Robau-Sánchez, A., A.
Aguilar-Elguézabal and J. Aguilar-Pliego, “Chemical activation of quercus agrifolia
char using KOH: Evidence of cyanide presence,” Microporous and Mesoporous
Materials, 85, 331-339 (2005).
Salviulo, G., M. Bettinelli,
U. Russo, A. Speghini and L. Nodari, “Synthesis and structural characterization
of Fe3+-doped calcium hydroxyapatites: Role of precursors and synthesis method,”
J. Mater.
Sci., 46, 910-922 (2011).
Sicupira, D.C., T. Tolentino
Silva, V.A. Leão and M.B. Mansur, “Batch removal of manganese from acid mine
drainage using bone char;” Braz. J. Chem. Eng., 31, 195-204
(2014).
Singh, A., “Hydroxyapatite,
a biomaterial: Its chemical synthesis, characterizationand study of biocompatibility
prepared from shell of garden snail,” Bull. Mater. Sci., 35, 1031-1038
(2012).
Sobczak, A., Z. Kowalski
and Z. Wzorek, “Preparation of hydroxyapatite from animal bones,” Acta Bioeng.
Biomech., 11, 23-28 (2009).
Sobczak-Kupiec, A.,
Z. Wzorek, R. Kijkowska and Z. Kowalski, “Effect of calcination conditions of
pork bone sludge on behaviour of hydroxyapatite in simulated body fluid,” Bull.
Mater. Sci., 36, 755-764 (2013).
Tanaka, H., T. Watanabe
and M. Chikazawa, “FTIR and TPD studies on the adsorption of pyridine,
n-butylamineand acetic acid on calcium hydroxyapatite,” J. Chem. Soc.,
Faraday Trans., 93, 4377-4381 (1997).
Wang, Y. and W.J. Thomson,
“The effect of sample preparation on the thermal decomposition of CaCO3,” Thermochimica Acta,
255,
383-390
(1995).
Received: January 8, 2016.
Sent to Subject Editor: April 27, 2016.
Accepted: September 29, 2016.
Recommended by Subject Editor: Orlando Alfano